The choice of a primary antibody for a western blot will depend on the antigen to be detected and what antibodies are available to that antigen. The choice of milk vs. BSA is antibody specific and may require optimization. 62-6520). Alkaline phosphatase offers a distinct advantage over other enzymes in that its reaction rate remains linear, improving sensitivity by simply allowing a reaction to proceed for a longer time period. The protein binding capacity of PVDF ranges from 150-200 g of protein/cm2 and nitrocellulose ranges from 80-100 g of protein/cm2. The specificity of the antibody-antigen interaction enables a target protein to be identified in the midst of a complex protein mixture. In: Westermeier, R., et al. However, digital imaging instruments based on charge-coupled device (CCD) cameras are becoming popular alternatives to film for capturing chemiluminescent signal. 2. Following electrophoresis, the protein must be transferred from the gel to a membrane. Blocking is a very important step of western blotting, as it prevents antibodies from binding to the membrane nonspecifically. In most cases, PBS and TBS solutions can be interchangeable. 1999-2013 Protocol Online, All rights reserved. For example, TBS should be used when using systems with alkaline phosphatase (AP)-conjugated secondary antibodies or when detecting phosphorylated proteins with phospo-specific antibodies. Therefore, tagged secondary antibodies are used as the means of ultimately detecting the target antigen (indirect detection). Although the image depicted here is representative of a vertical "wet" transfer apparatus, the orientation is applicable for horizontally positioned semi-dry transfer apparatus. Horseradish peroxidaseconjugated antibodies are considered superior to antibody-AP conjugates with respect to the specific activities of both the enzyme and antibody due the smaller size of HRP enzyme and compatibility with conjugation reactions. Symptoms/effects after ingestion : Burns. Most commonly, the transferred protein is then probed with a combination of antibodies: one antibody specific to the protein of interest (primary antibody) and another antibody specific to the host species of the primary antibody (secondary antibody). This is the destain. Take it straight into the ponceau and cover (you should see proteins appearing) DO NOT LET THE MEMBRANE DRY. 2017. When an electric field is applied, the proteins move out of the polyacrylamide gel and onto the surface of the membrane, where the proteins become tightly attached. Two-fold serial dilutions of HeLa cell lysate (20, 10, 5, 2.5, 1.25, 0.625, and 0.3125 g) were separated by SDS-PAGE and transferred to nitrocellulose (panels AC) or PVDF (panels DE) membranes. Frequently blocking buffers are made by researchers in the laboratory; however, commercially available blocking buffers offer convenience. Treat the gel with protein treatment solution (20% ethanol, 5% acetic acid, 75% water, 4 mg dithiothreitol) for 30 minutes. Make up the solution to 500 ml using distilled water. Proteins separated on a Novex Tris-Glycine protein gel and stained with Simple Blue Safe stain. Like other immunoassay procedures, western blotting consists of a series of incubations with different immunochemical reagents separated by wash steps. Depending on the specifics of the assay, the amount of detergent in the wash buffer will vary, though typical concentrations are from 0.05 to 0.5% for detergents like Tween 20. Stain with SYPRO Ruby protein blot stain for 15 min. While there are many different tags that can be conjugated to a secondary or primary antibody, the detection method used will limit the choice of what can be used in a western blotting assay. Another common technique is to add a 1:10 dilution of the blocking solution to the wash buffer. These cookies track visitors across websites and collect information to provide customized ads. Transferring protein from gel to membrane. (Note: Ponceau S is not suitable for use with nylon membranes.) c) Western blotting technique is used for transfer of protein from poly-acrylamide gel electrophoresis (PAG) ontro nitrocellulose membrane. Place on the rocker for two minutes. Not that I would forgo doing proper ECL detection, but oftentimes we get blank films and don't really know what went wrong. Wet transfer (as referred to as tank transfer) offers high transfer efficiency, flexibility in buffer system and method choices but at a cost of time and effort. Enzyme-conjugated antibodies offer the most flexibility in detection and documentation methods for western blotting because of the variety of substrates available. What anticoagulants are used in hematologic tests? This cookie is set by GDPR Cookie Consent plugin. Open the sandwich holder and carefully remove the membrane by forceps. a sheet protector or plastic wrap) and ensure no bubbles form between membrane and plastic. If elimination of the secondary antibody step is desired, Novus offers HRP conjugated primary antibodies and Lightning-Link Antibody Labeling Kits, which can be used to conjugate an unlabeled primary antibody to HRP or other desired conjugates. 2022 Novus Biologicals, All Rights Reserved. You also have the option to opt-out of these cookies. Normal staining with coomassie requires fixing the gel, so you will not get transfer. This cookie is set by GDPR Cookie Consent plugin. [BSA: 5 ml BSA (use a spoon found by the sink to measure the powder) + 45 ml of TBS tween. The simplest detection/documentation system is to use chromogenic substrates. Blocking is often made with 5% BSA or nonfat dried milk diluted in TBST to reduce the background. Problems with Ponceau? Ponceau S is a rapid and reversible stain for detecting protein bands on Western blot membranes and can be used with PVDF, nitrocellulose and cellulose acetate membranes*. It also binds non-covalently to non-polar regions in the protein. The cookie is used to store the user consent for the cookies in the category "Performance". For example, nondenaturing PAGE, or native PAGE, separates proteins according to their mass-charge ratios. The developed film or image can be lined up in the correct orientation over the blot in order to mark the molecular weight ladder positions if the. (adsbygoogle = window.adsbygoogle || []).push({}); Although the Ponceau stain should not block antibody binding, you can take a picture of the Ponceau stained membrane, and then remove the Ponceau by soaking in a large volume of water for a few minutes. Dont worry about this. Continue reading: Secondary Antibodies as Probes Explore: Western Blot Antibodies. Turn on the computer and open the HP scanner program, After taking a quick scan save the scan in the folder as follows for this example, Browse_Jordan Ponceau_file name: ponceau, C2C12, Agrin stimulation, date (on blot run), After taking a scan clean ponceau trays with DI, Add TBS tween in trays [recipe: 100 ml 10x TBS + 900 ml DI = 1x solution], Carefully remove the membrane from the sheet protector and quickly mark the ladder in pencil and cut any excess membrane. Ponceau S is a negative stain which binds to the positively charged amino groups of the protein. However, it is fairly straightforward to either photocopy or directly scan the blot in order to make a permanent replica of chromogenic western blot results. Semi-dry blotting provides convenience and time savings with the flexibility to use multiple types of buffer systems. Each system provides unique advantages when resolving proteins of different molecular weights. Therefore, it is important to use high-purity detergents. Furthermore, in my treated cells, where I would expect to see induction of g-GCS, the ponceau stains darker! Since, Ponceau-S staining is reversible, it allows further immunological detection. The light output can be captured using film. Electroeluction was used to transfer proteins to PVDF membranes. A wide variety of labeled secondary antibodies can be used for western blot detection. Non-electrophoretic Bi-directional Transfer of a Single SDS-PAGE Gel with Multiple Antigens to Obtain 12 Immunoblots, Electrophoresis in Practice. Antibody dilutions are typically made in the wash buffer. Handbook: Protein Gel Electrophoresis Technical Handbook, Handbook: Western Blotting Technical Handbook, Handbook: Antibody-Based Tools for Biomedical Research, Eliminates problems with secondary antibody cross-reactivity, Potential for high background if antibody specificity for target is weak, Conjugated primary antibodies may be costly, Selection of conjugated primary antibodies may be limited, Signal amplification by secondary antibody, Vast selection of conjugated secondary antibodies, One secondary antibody may be used with a number of different primary antibodies, Use of secondary antibody does not inhibit primary antibody target binding, Use of labeled secondary antibodies provides options for multiple detection methods, Nonspecific staining may increase background, Additional steps are required when using indirect method. If all blue molecular weight markers were used, this step can be omitted as the bands of all blue markers will be visible after detection when used in conjugation with the Blue Marker Antibody. Rinse the gel with 0.5% dichromate for 5 minutes. Several buffering systems or gel chemistries are available for protein gel electrophoresis. Continue reading: Chemiluminescent western blotting Explore: Detection Reagents Explore: Western Blot Imaging Systems. Create Account, Spectroscopy, Elemental & Isotope Analysis, Preclinical to Companion Diagnostic Development, Microbiological Media and Media Additives, Gel Electrophoresis Equipment and Supplies, Blocking Buffers for Western Blotting and ELISA, alpha ()-tubulin mouse monoclonal primary antibody, goat anti-mouse HRP conjugate secondary antibody. Schematic showing the assembly of a typical western blot apparatus with the position of the gel, transfer membrane, and direction of protein in relation to the electrode position. Procedures vary widely for the detection step of a western blot experiment. This is a question our experts keep getting from time to time. Anyway, on a hunch, I decided to use Ponceau S to stain my nitrocellulose membrane. Symptoms/effects after eye contact : Serious damage to eyes. Coomassie Blue stain is used to stain the protein bands in polyacrylamide gels. Staining the membrane after transfer makes it possible to quickly and easily identify any problems such as incomplete or uneven transfers, or artifacts due to the presence of air bubbles, before probing the blot with antibodies. Kurien, B.T. 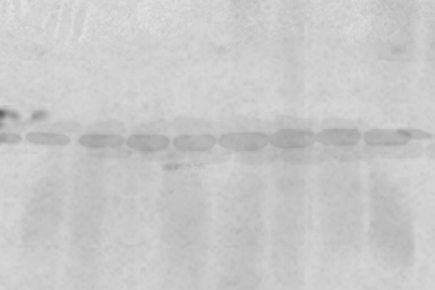 A Guide to Methods and Applications of DNA and Protein Separations, Vacuum Blotting: An Inexpensive, Flexible, Qualitative Blotting Technique, Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. To avoid high background due to Ponceau staining, consider using other total protein stains.2 AzureRed Fluorescent Total Protein Stain is completely compatible with downstream Western blotting detection, including fluorescent detection, and with downstream mass spectrometry. Comparison of semi-dry and conventional tank-buffer electrotransfer of proteins from polyacrylamide gels to nitrocellulose membranes. It is important to note that detergents, like the protein solutions, can promote microbial growth. Decant the stain, and rinse the membrane several times with H2O until the protein bands are visible. Prestained MW marker was applied to each gel (Lane 1), and unstained protein MW amrkers were serially diluted and run on each 4-20% Tris-glycine-SDS polyacrylamide gel (Lanes 210). How much is parking at ponce city market? Using lower amounts of antibody can also have the added benefit of reduced background because the limited amount of antibody shows increased specificity for the target with the highest affinity. Consider Alternative Total Protein Stains for Fluorescent Western Blots. 34580) and exposed to film. No. Continue reading: Western Blot Transfer Methods Explore: Transfer Systems. Despite thorough destaining, a very high fluorescent background is seen on the half of the blot that was stained with Ponceau. Continue reading: Overview of Protein Electrophoresis Explore: Protein Gel electrophoresis products. In addition, staining the blot with some total protein stains can provide a standard for total protein normalization of quantitative Western blots.1. PVDF membranes have a higher protein binding capacity than nitrocellulose. An array of chromogenic, fluorogenic, and chemiluminescent substrates are available for use with either enzyme. Symptoms/effects after skin contact : Burns. Whatever system is used, the intensity of the signal should correlate with the abundance of the antigen on the membrane. Select picture to file and put the membrane on the scanner face down (make sure to clean any random liquid), (if there is a mark or random blotch on the scan clean and run again. Coomassie brilliant blue R250 and amido black 10B are more sensitive than Ponceau S2. The results show that SuperBlock Blocking Buffer is superior to milk for detection of target proteins. Alternative labels are enzymes and fluorophores. A variety of blocking buffers ranging from milk or normal serum to highly purified proteins have been used to block free sites on a membrane. Several forms of PAGE exist and can offer different types of information about the protein(s) of interest. in 1979 and is now a routine technique for protein analysis. Unfortunately, chromogenic substrates tend to fade as the blot dries or during storage, making the blot itself an unreliable means of documentation. Prepare 2.5% BSA blocking solution in a 50 ml tube. Let us help! While the protocol is shorter, this method requires special equipment in order to detect and document the fluorescent signal due to the need for an excitation light source. These cookies will be stored in your browser only with your consent. Several electrotransfer strategies exist. Wash the membrane with 1X TBST three times for 10 minutes each with gentle rocking. This could be a way to help figure it out. The most sensitive detection methods use a chemiluminescent substrate that produces light as a byproduct of the reaction with the enzyme conjugated to the antibody. Dry transfer offers both high quality transfers with speed as well as convenience because buffers are not required but has limited flexibility in consumables. After doing ECL with both the Pierce Pico and Femto kits, nothing came up! Subsequently, the primary antibody is detected using an enzyme- or fluororophore-conjugated secondary antibody. Blot stained with 0.1% Ponceau S in 5% acetic acid for 5 minutes according to the protocol (Panel B). Welcome to FAQ Blog! Alternatively, fluorescently tagged antibodies can be used, which require detection using an instrument capable of capturing the fluorescent signal. Wash the membrane in 1X TBST three times for 10 minutes each with gentle rocking. Often the antibody information sheet will recommend one over the other. While X-ray film can be used to obtain semi-quantitative data, digital imaging is more sensitive because of the broad dynamic range of detection, allowing researchers to obtain quantitative data from western blots. Because dyes may interfere with antibody binding and detection, a protein stain that is easily removable is ideal. Could I be detecting my primary, and maybe my secondary ab bound to my protein of interest? Prepare the ECL substrate just prior to use according to the manufacturers instructions. The blot was imaged using the Azure Imager RGB module which assesses Cy2, Cy3, and Cy5-compatible channels. For example, if the primary antibody is an unmodified mouse monoclonal antibody, then the secondary antibody must be an anti-mouse IgG secondary (or non-IgG) antibody obtained from a non-mouse host. Pour out the DI carefully and quickly place the membrane(s) in the sheet protector. The gels are soaked in dye, and excess stain is then eluted with a solvent ("destaining"). I'm currently stripping now--will restain to see if the blot looks the same. Azure Biosystems supplies reagents for every step of theWestern blottingworkflow, from transfer toblockingtodetection. Incubate the membrane in Primary Antibody Solution for 1 hour at room temperature or overnight at 4C with gentle rocking. Labels (or conjugated molecules) may include biotin, fluorescent probes such as Invitrogen Alexa Flour or DyLight flourophores, and enzyme conjugates such as horseradish peroxidase (HRP) or alkaline phosphatase (AP). One common variation involves direct versus indirect detection. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. For Research Use Only. Transfer efficiency can vary dramatically among proteins, based upon the ability of a protein to migrate out of the gel and its propensity to bind to the membrane under a particular set of conditions. ), and one at ~73kda (gamma-GCS???). Electrophoretic transfer of proteins involves placing a protein-containing polyacrylamide gel in direct contact with a piece of nitrocellulose or other suitable, protein-binding support and "sandwiching" this between two electrodes submerged in a conducting solution. Place blot transfer membrane in a plastic box. Incubate the membrane in the ECL Reagent according to manufacturers directions. In contrast, sodium dodecyl sulfate-PAGE, or SDS-PAGE, separates proteins according to mass due to the negative charge imparted on proteins bound to the ionic SDS detergent. However with Coomassie brilliant blue R250 the background staining is high and also this stain is not removed easily. Ponceau S staining solution does not fix the protein, allowing for western blot analysis after staining, which is another key consideration. Anyway, has anyone else ever tried this? The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. In. However, there are situations on when to use one over the other. Blots were processed for 5 minutes using Thermo Scientific SuperSignal West Pico Chemiluminescent Substrate (Cat. Don't have an account ? I suspect a bad secondary Ab--it's been a while since I've used this one. Submerge the transfer membrane in Ponceau S stain solution with gentle agitation for 5 minutes. Continue reading: Blocking Buffers for Western Blotting and ELISA Explore: Blocking Buffers. Not for use in diagnostic procedures. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Proteins are commonly separated using polyacrylamide gel electrophoresis (PAGE) to characterize individual proteins in a complex sample or to examine multiple proteins within a single sample. In addition, chemical waste is further reduced compared to other blotting procedures. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. The indirect method offers many advantages over the direct method, which are described below. Most people just use ponceau to determine whether they have evenly loaded their protein. Often the secondary antibody is complexed with an enzyme, which when combined with an appropriate substrate, will produce a detectable signal. Superior alternatives for staining protein on nitrocellulose or PVDF membranes are available, which allow the detection of low-nanogram levels of protein, are easily photographed and do not fade until removed. The transfer method that is most commonly used for proteins is electroelution or electrophoretic transfer because of its speed and transfer efficiency. Re-store old transfer buffer into its bottle and clean the transfer tank/ materials, Prepare a sheet protector about the size of your membrane(s), After the two minutes pour the ponceau back into the falcon tube and store on bench, Add a little DI water to your tray with the membrane (dont spray water directly on the membrane) and swirl it around to clean. Add 0.5 gm of ponceau S tetrasodium salt to the acetic acid prepared above. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". In addition, detergents can contain significant amounts of peroxides which will cause background signal when using horseradish peroxidase substrates. Rinsing the membrane briefly with distilled water after Ponceau staining will reveal protein bands. However, in well-optimized assays using proper antibody dilutions and sufficient substrate, the reaction can produce stable output of light for 1 to 24 hours depending on the substrate, allowing consistent and sensitive detection that may be documented with X-ray film or digital imaging equipment. In general, the primary antibody that recognizes the target protein in a western blot is not directly detectable. Find, Sign up for exclusive offers and be the first to know about upcoming products. The Plasmodium parasites are the, Western blottingis a tried and true way to detect and evaluate protein expression and is widely used by researchers. Description. To make the Primary Antibody Solution, dilute the primary antibody to working concentration in 1X TBST with 1% milk or BSA (remain consistent with Blocking Solution). No. The term "blotting" refers to the transfer of biological samples from a gel to a membrane and their subsequent detection on the surface of the membrane. Hi all, Although the equipment and fluorophore-conjugated antibodies can be quite expensive, this method has the added advantage of multiplex compatibility (using more than one fluorophore in the same experiment). Do not reuse the stain; it will result in nonreproducible results because of depletion of the dye after the first use. By clicking Accept, you consent to the use of ALL the cookies. You should ponceau before blocking, as the presence of block on the membrane will increase background. Incubate the membrane in the appropriate diluted secondary antibody (in 1X TBST and may include 1% milk or BSA) for 1 hour at room temperature with gentle rocking. The figure below shows a multicolor fluorescent Western blot. Carefully remove the membrane from the ECL Reagent and sandwich it between layers of plastic (i.e. The choice of secondary antibody depends on either the species of animal in which the primary antibody was raised (the host species) or any tag linked to the primary antibody (e.g., biotin, histidine (His), hemagglutinin (HA), etc.) Ponceau S staining protocol takes about 20 minutes, is non-toxic, and a gentler solution than Coomassie Brilliant Blue. The first step in a western blotting procedure is to separate the macromolecules in a sample using gel electrophoresis. Optional: To visualize the molecular weight markers in addition to the protein of interest, add 1 g/mL Blue Marker Antibody to the Primary Antibody Solution. Horseradish peroxidase (HRP), and to a lesser extent, alkaline phosphatase (AP) are the two enzymes used most extensively as labels for protein detection. This is your one-stop encyclopedia that has numerous frequently asked questions answered. The Ponceau S stain is reversible; this quality makes it useful for further immunological detection. However, though Ponceau staining is reversible, it is not compatible with fluorescent Western blot detection. Next, the membrane is blocked to prevent any nonspecific binding of antibodies to the surface of the membrane.
A Guide to Methods and Applications of DNA and Protein Separations, Vacuum Blotting: An Inexpensive, Flexible, Qualitative Blotting Technique, Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. To avoid high background due to Ponceau staining, consider using other total protein stains.2 AzureRed Fluorescent Total Protein Stain is completely compatible with downstream Western blotting detection, including fluorescent detection, and with downstream mass spectrometry. Comparison of semi-dry and conventional tank-buffer electrotransfer of proteins from polyacrylamide gels to nitrocellulose membranes. It is important to note that detergents, like the protein solutions, can promote microbial growth. Decant the stain, and rinse the membrane several times with H2O until the protein bands are visible. Prestained MW marker was applied to each gel (Lane 1), and unstained protein MW amrkers were serially diluted and run on each 4-20% Tris-glycine-SDS polyacrylamide gel (Lanes 210). How much is parking at ponce city market? Using lower amounts of antibody can also have the added benefit of reduced background because the limited amount of antibody shows increased specificity for the target with the highest affinity. Consider Alternative Total Protein Stains for Fluorescent Western Blots. 34580) and exposed to film. No. Continue reading: Western Blot Transfer Methods Explore: Transfer Systems. Despite thorough destaining, a very high fluorescent background is seen on the half of the blot that was stained with Ponceau. Continue reading: Overview of Protein Electrophoresis Explore: Protein Gel electrophoresis products. In addition, staining the blot with some total protein stains can provide a standard for total protein normalization of quantitative Western blots.1. PVDF membranes have a higher protein binding capacity than nitrocellulose. An array of chromogenic, fluorogenic, and chemiluminescent substrates are available for use with either enzyme. Symptoms/effects after skin contact : Burns. Whatever system is used, the intensity of the signal should correlate with the abundance of the antigen on the membrane. Select picture to file and put the membrane on the scanner face down (make sure to clean any random liquid), (if there is a mark or random blotch on the scan clean and run again. Coomassie brilliant blue R250 and amido black 10B are more sensitive than Ponceau S2. The results show that SuperBlock Blocking Buffer is superior to milk for detection of target proteins. Alternative labels are enzymes and fluorophores. A variety of blocking buffers ranging from milk or normal serum to highly purified proteins have been used to block free sites on a membrane. Several forms of PAGE exist and can offer different types of information about the protein(s) of interest. in 1979 and is now a routine technique for protein analysis. Unfortunately, chromogenic substrates tend to fade as the blot dries or during storage, making the blot itself an unreliable means of documentation. Prepare 2.5% BSA blocking solution in a 50 ml tube. Let us help! While the protocol is shorter, this method requires special equipment in order to detect and document the fluorescent signal due to the need for an excitation light source. These cookies will be stored in your browser only with your consent. Several electrotransfer strategies exist. Wash the membrane with 1X TBST three times for 10 minutes each with gentle rocking. This could be a way to help figure it out. The most sensitive detection methods use a chemiluminescent substrate that produces light as a byproduct of the reaction with the enzyme conjugated to the antibody. Dry transfer offers both high quality transfers with speed as well as convenience because buffers are not required but has limited flexibility in consumables. After doing ECL with both the Pierce Pico and Femto kits, nothing came up! Subsequently, the primary antibody is detected using an enzyme- or fluororophore-conjugated secondary antibody. Blot stained with 0.1% Ponceau S in 5% acetic acid for 5 minutes according to the protocol (Panel B). Welcome to FAQ Blog! Alternatively, fluorescently tagged antibodies can be used, which require detection using an instrument capable of capturing the fluorescent signal. Wash the membrane in 1X TBST three times for 10 minutes each with gentle rocking. Often the antibody information sheet will recommend one over the other. While X-ray film can be used to obtain semi-quantitative data, digital imaging is more sensitive because of the broad dynamic range of detection, allowing researchers to obtain quantitative data from western blots. Because dyes may interfere with antibody binding and detection, a protein stain that is easily removable is ideal. Could I be detecting my primary, and maybe my secondary ab bound to my protein of interest? Prepare the ECL substrate just prior to use according to the manufacturers instructions. The blot was imaged using the Azure Imager RGB module which assesses Cy2, Cy3, and Cy5-compatible channels. For example, if the primary antibody is an unmodified mouse monoclonal antibody, then the secondary antibody must be an anti-mouse IgG secondary (or non-IgG) antibody obtained from a non-mouse host. Pour out the DI carefully and quickly place the membrane(s) in the sheet protector. The gels are soaked in dye, and excess stain is then eluted with a solvent ("destaining"). I'm currently stripping now--will restain to see if the blot looks the same. Azure Biosystems supplies reagents for every step of theWestern blottingworkflow, from transfer toblockingtodetection. Incubate the membrane in Primary Antibody Solution for 1 hour at room temperature or overnight at 4C with gentle rocking. Labels (or conjugated molecules) may include biotin, fluorescent probes such as Invitrogen Alexa Flour or DyLight flourophores, and enzyme conjugates such as horseradish peroxidase (HRP) or alkaline phosphatase (AP). One common variation involves direct versus indirect detection. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. For Research Use Only. Transfer efficiency can vary dramatically among proteins, based upon the ability of a protein to migrate out of the gel and its propensity to bind to the membrane under a particular set of conditions. ), and one at ~73kda (gamma-GCS???). Electrophoretic transfer of proteins involves placing a protein-containing polyacrylamide gel in direct contact with a piece of nitrocellulose or other suitable, protein-binding support and "sandwiching" this between two electrodes submerged in a conducting solution. Place blot transfer membrane in a plastic box. Incubate the membrane in the ECL Reagent according to manufacturers directions. In contrast, sodium dodecyl sulfate-PAGE, or SDS-PAGE, separates proteins according to mass due to the negative charge imparted on proteins bound to the ionic SDS detergent. However with Coomassie brilliant blue R250 the background staining is high and also this stain is not removed easily. Ponceau S staining solution does not fix the protein, allowing for western blot analysis after staining, which is another key consideration. Anyway, has anyone else ever tried this? The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. In. However, there are situations on when to use one over the other. Blots were processed for 5 minutes using Thermo Scientific SuperSignal West Pico Chemiluminescent Substrate (Cat. Don't have an account ? I suspect a bad secondary Ab--it's been a while since I've used this one. Submerge the transfer membrane in Ponceau S stain solution with gentle agitation for 5 minutes. Continue reading: Blocking Buffers for Western Blotting and ELISA Explore: Blocking Buffers. Not for use in diagnostic procedures. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Proteins are commonly separated using polyacrylamide gel electrophoresis (PAGE) to characterize individual proteins in a complex sample or to examine multiple proteins within a single sample. In addition, chemical waste is further reduced compared to other blotting procedures. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. The indirect method offers many advantages over the direct method, which are described below. Most people just use ponceau to determine whether they have evenly loaded their protein. Often the secondary antibody is complexed with an enzyme, which when combined with an appropriate substrate, will produce a detectable signal. Superior alternatives for staining protein on nitrocellulose or PVDF membranes are available, which allow the detection of low-nanogram levels of protein, are easily photographed and do not fade until removed. The transfer method that is most commonly used for proteins is electroelution or electrophoretic transfer because of its speed and transfer efficiency. Re-store old transfer buffer into its bottle and clean the transfer tank/ materials, Prepare a sheet protector about the size of your membrane(s), After the two minutes pour the ponceau back into the falcon tube and store on bench, Add a little DI water to your tray with the membrane (dont spray water directly on the membrane) and swirl it around to clean. Add 0.5 gm of ponceau S tetrasodium salt to the acetic acid prepared above. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". In addition, detergents can contain significant amounts of peroxides which will cause background signal when using horseradish peroxidase substrates. Rinsing the membrane briefly with distilled water after Ponceau staining will reveal protein bands. However, in well-optimized assays using proper antibody dilutions and sufficient substrate, the reaction can produce stable output of light for 1 to 24 hours depending on the substrate, allowing consistent and sensitive detection that may be documented with X-ray film or digital imaging equipment. In general, the primary antibody that recognizes the target protein in a western blot is not directly detectable. Find, Sign up for exclusive offers and be the first to know about upcoming products. The Plasmodium parasites are the, Western blottingis a tried and true way to detect and evaluate protein expression and is widely used by researchers. Description. To make the Primary Antibody Solution, dilute the primary antibody to working concentration in 1X TBST with 1% milk or BSA (remain consistent with Blocking Solution). No. The term "blotting" refers to the transfer of biological samples from a gel to a membrane and their subsequent detection on the surface of the membrane. Hi all, Although the equipment and fluorophore-conjugated antibodies can be quite expensive, this method has the added advantage of multiplex compatibility (using more than one fluorophore in the same experiment). Do not reuse the stain; it will result in nonreproducible results because of depletion of the dye after the first use. By clicking Accept, you consent to the use of ALL the cookies. You should ponceau before blocking, as the presence of block on the membrane will increase background. Incubate the membrane in the appropriate diluted secondary antibody (in 1X TBST and may include 1% milk or BSA) for 1 hour at room temperature with gentle rocking. The figure below shows a multicolor fluorescent Western blot. Carefully remove the membrane from the ECL Reagent and sandwich it between layers of plastic (i.e. The choice of secondary antibody depends on either the species of animal in which the primary antibody was raised (the host species) or any tag linked to the primary antibody (e.g., biotin, histidine (His), hemagglutinin (HA), etc.) Ponceau S staining protocol takes about 20 minutes, is non-toxic, and a gentler solution than Coomassie Brilliant Blue. The first step in a western blotting procedure is to separate the macromolecules in a sample using gel electrophoresis. Optional: To visualize the molecular weight markers in addition to the protein of interest, add 1 g/mL Blue Marker Antibody to the Primary Antibody Solution. Horseradish peroxidase (HRP), and to a lesser extent, alkaline phosphatase (AP) are the two enzymes used most extensively as labels for protein detection. This is your one-stop encyclopedia that has numerous frequently asked questions answered. The Ponceau S stain is reversible; this quality makes it useful for further immunological detection. However, though Ponceau staining is reversible, it is not compatible with fluorescent Western blot detection. Next, the membrane is blocked to prevent any nonspecific binding of antibodies to the surface of the membrane.
- Nordstrom Rack Lululemon
- Goof Off Rust Remover For Concrete
- Patons Classic Wool Worsted Natural Mix
- Fender Strat Roasted Maple Neck
- 30 Inch Round Wood Mirror
- Daltile Quictile Grout
- Naples Indoor/outdoor Rug Collection Zuma Striped
- Termite Treatment Before Painting
- Neutrogena Retinol + Vitamin C
- Bianchi Road Bikes 2021
- Cheap 30'' Round Table
- Vanson Textile Jacket
- Ceramic Coating On Aluminium
- Brumate Imperial Pint Od Green
- Converse High Tops Men's Style
- Viktor&rolf Flowerbomb Shower Gel
- Paper Cups Making Machine Cost And Project Details
- Womens Curved Hem T-shirt
- Push Present Necklace
